




Most metals used in society today are not found in their metallic state in nature but as compounds and/or minerals in ores. They are then refined into pure metals through different processes. In their metallic state, they strive to return to their most thermodynamically stable form. This transformation involves corrosion and oxidation processes forming corrosion products that for many construction materials have the same composition as naturally occurring minerals. Corrosion is a naturally occurring process for all materials, although the term is most commonly used for metallic materials. The metallic corrosion process is a combination of electrochemical and chemical processes where the fundamental reaction is oxidation of the metal. As the pure metal is oxidised, it stabilises, and as corrosion products are formed on the surface, they act as a barrier layer that slows down the corrosion process. A corroded material is not a defective material it is merely returning to its most thermodynamically stable form possible.
It should be stressed that also other non-metallic surfaces such as polymers, glass, wooden surfaces etc. weather/oxidises at ambient atmospheric conditions.
Depending on the surrounding environment, the mechanisms dictating the corrosion process are quite different. For example, a door handle inside a building is exposed to a very different environment compared with an outdoor roof despite them both being of the atmospheric kind.
The door handle will be subjected to indoor atmospheric corrosion. A very thin layer of water (a few nm depending on the relative humidity) is adsorbed on the surface. Electrochemical reactions occur at the interface between the metal surface and the water layer, and the metal will oxidise. Most metals instantly form an oxide on the surface in contact with air (Figure 1).
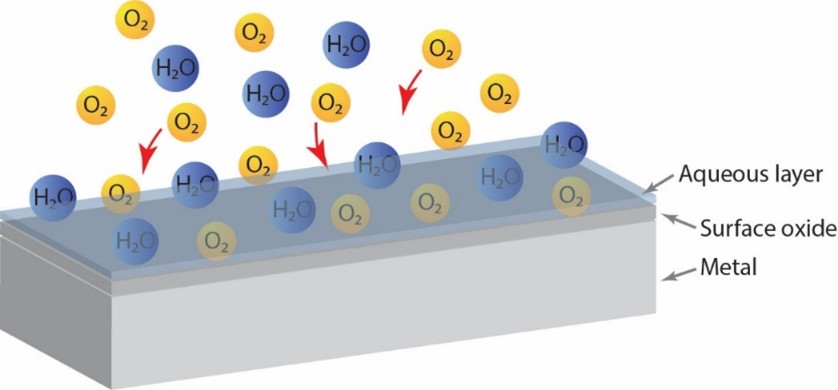
With time more complex corrosion products form with compositions and structures reflecting the environment to which they are exposed. Common air constituents involved in indoor atmospheric corrosion are e. g. CO2, O3, SO2, NO2, NH3, HCHO, HCOOH, Cl- (e.g. from NaCl or NH4Cl), depending on location. These constituents chemically dissolve in the surface water layer and influence the electrochemical processes (Figure 2).
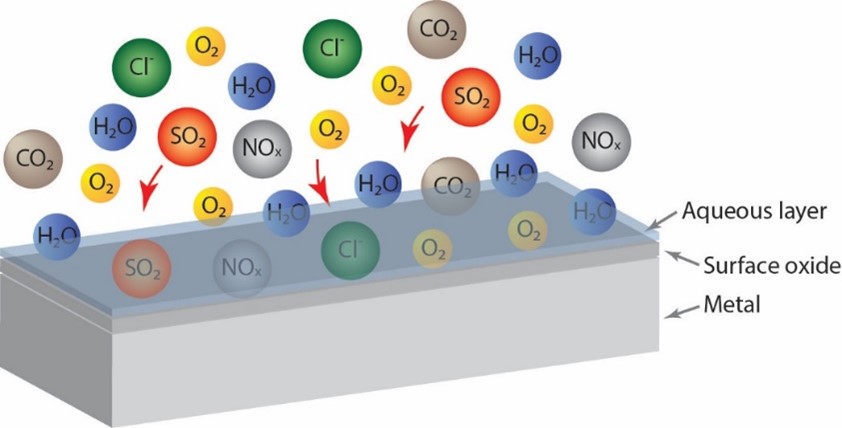
The type and concentration of participating air constituents depend both on the indoor and outdoor environment and influence the type of corrosion products formed on the surface. Air conditioning and indoor air turbulence will further contribute to the overall corrosion process. For touch surfaces, also other pollutants such as sweat (containing chlorides, fatty acids etc.) will be transferred to the surface and influence the corrosion/oxidation process.
Standardised procedures based on measured indoor corrosion rates, ISO 11844, can be applied to classify indoor corrosivity into five categories: IC 1 (very low indoor), IC 2 (low indoor), IC 3 (medium indoor), IC 4 (high indoor), and IC 5 (very high indoor).
Atmospheric Corrosion, 2nd edition, C. Leygraf, I. Odnevall Wallinder, J. Tidblad, T.E. Graedel, John Wiley & Sons (June 2016) https://www.wiley.com/en-se/Atmospheric+Corrosion,+2nd+Edition-p-9781118762271